Abstract
Despite the recent progress in Zn-air battery fabrication, the development of highly electroactive, non-precious-metal-based, and durable electrocatalysts for such batteries remains challenging. To address this issue, spinel-type MnCo2O4.5 nanoparticles (NPs) prepared by a solvothermal method followed by calcination were characterized by a range of instrumental techniques and employed in rechargeable Zn-air batteries to promote the oxygen reduction and oxygen evolution reactions. The bifunctional activity of the MnCo2O4.5 NPs was further evaluated by linear sweep voltammetry, which showed that both the reduction and oxidation current densities obtained in the presence of this catalyst exceed those observed with catalyst-free carbon. Moreover, the incorporation of the MnCo2O4.5 NPs into an air-breathing cathode resulted in a decreased charge–discharge voltage gap and improved round-trip efficiency. Therefore, these MnCo2O4.5 NPs are a highly beneficial and novel kind of bifunctional electrocatalyst for rechargeable Zn-air batteries.
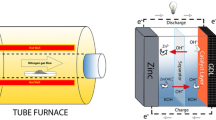
Graphic abstract
Schematic illustration of solvothermal synthesis, bifunctional catalytic activity of MnCo2O4.5 nanoparticles for Zn-air batteries.







Similar content being viewed by others
References
Sun D, Jin G, Wang H, Huang X, Ren Y, Jiang J, He H, Tang Y (2014) LixV2O5/LiV3O8 nanoflakes with significantly improved electrochemical performance lithium-ion batteries. J Mater Chem A 2:8009–8016. https://doi.org/10.1039/c4ta00868e
Sun D, Jin G, Wang H, Liu P, Ren Y, Jiang Y, Tang Y, Huang X (2014) Aqueous rechargeable lithium batteries using NaV6O15 nanoflakes as high-performance anodes. J Mater Chem A 2:12999–13005. https://doi.org/10.1039/c4ta01675k
Du Z, Wood DL, Daniel C, Kalnaus S, Li J (2017) Understanding limiting factors in the thick electrode performance as applied to high energy density Li-ion batteries. J Appl Electrochem 47:405–415. https://doi.org/10.1007/s10800-017-1047-4
Li X, Zhang Y, Su Z, Zhao Y, Zhao X, Wang R (2017) Graphene nanosheets as backbone to build a 3D conductive network for negative active materials of lead-acid batteries. J Appl Electrochem 47:619–630. https://doi.org/10.1007/s10800-017-1067-0
Min YJ, Oh SJ, Kim MS, Choi JH, Eom S (2018) Effect of carbon properties on the electrochemical performance of carbon-based air electrodes for rechargeable zinc-air batteries. J Appl Electrochem 48:405–413. https://doi.org/10.1007/s10800-018-1173-7
Davari E, Ivey DG (2017) Synthesis and electrochemical performance of manganese nitride as an oxygen reduction and oxygen evolution catalyst for zinc-air secondary batteries. J Appl Electrochem 47:815–827. https://doi.org/10.1007/s10800-017-1084-z
Li Y, Gong M, Liang Y, Feng J, Kim JE, Wang H, Hong G, Zhang B, Dai H (2013) Advanced zinc-air batteries based on high-performance hybrid electrocatalyst. Nat Commun 4:1805. https://doi.org/10.1038/ncomms2812
Harnish F, Scholder U (2010) From MFC to MXC: chemical and biological cathode and their potential for microbial bioelectrochemical system. Chem Soc Rev 39:4433–4448. https://doi.org/10.1039/c003068f
Lee JS, Kim ST, Cao R, Choi NS, Liu M, Lee KT, Cho J (2011) Metal-air batteries with high energy density: lithium-air, versus Zn-air. Adv Energy Mater 1:34–50. https://doi.org/10.1002/aenm.201000010
Zhang X, Wang XG, Xie Z, Zhou Z (2016) Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ 1:4–17. https://doi.org/10.1016/j.gee.2016.04.004
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells. J Power Sources 180:683–694. https://doi.org/10.1016/j.jpowsour.2008.02.074
Wang X, Wang J, Wang D, Dou S, Ma Z, Wu J, Tao L, Shen A, Ouyang C, Liu Q, Wang S (2014) One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem Commun 50:4839–4842. https://doi.org/10.1039/c4cc00440j
Gong K, Du F, Xi Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764. https://doi.org/10.1126/science.1168049
Yang L, Jiang S, Zhao Y, Zhu L, Chen S, Wang X, Wu Q, Ma J, Ma Y, Hu Z (2011) Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew Chem Int Ed 50:7132–7135. https://doi.org/10.1126/science.1168049
Xiao M, Zhu J, Feng L, Liu C, Xing W (2015) Meso/microporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv Mater 27:2521–2527. https://doi.org/10.1002/adma.201500262
Liu L, Zeng G, Chen J, Bi L, Dai L, When Z (2018) N-doped carbon nanosheets as pH universal ORR electrocatalyst in various fuel cell devices. Nano Energy 49:393–402. https://doi.org/10.1016/j.nanoen.2018.04.061
Ye L, Chai G, Wen Z (2017) Zn-MOF-74-derived N-doped mesoporous carbon as pH-universal electrocatalyst for oxygen reduction reaction. Adv Funct Mater 27:1606190. https://doi.org/10.1002/adfm.201606190
Park MS, Kim J, Kim KJ, Lee JW, Kim JH, Yamauchi Y (2015) Porous nanoarchitectures of spinel-type transitional metal oxides electrochemical energy storage systems. Phys Chem Chem Phys 17:30963–30977. https://doi.org/10.1039/c5cp05936d
Cai P, Huang J, Chen J, When Z (2017) Oxygen-incorporated amorphous cobalt sulfide porous nanotubes as a highly active electrocatalysts for the oxygen evolution reaction in alkaline/neutral medium. Angew Chem Int Ed 56:1–5. https://doi.org/10.1002/anie.201701280
Osgood H, Devaguptapu SV, Xu H, Cho J, Wu G (2016) Transition metal (Fe Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 11:601–625. https://doi.org/10.1016/j.nantod.2016.09.001
Ma C, Xu N, Qiao J, Jian S, Zhang J (2016) Facile synthesis of NiCo2O4 nanosphere-carbon nanotubes hybrid as an efficient bifunctional electrocatalyst for rechargeable Zn-air batteries. Int J Hydrog Energy 41:9211–9218. https://doi.org/10.1016/j.ijhydene.2015.125.022
Wang X, Li Y, Jin T, Meng J, Jiao L, Zhu M, Chen J (2017) Electrospun thin-walled CuCo2O4@C nanotubes as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Nano Lett 17:7989–7994. https://doi.org/10.1021/acs.nanolett.7b04502
Liu ZQ, Cheng H, Li N, Ma TY, Su YZ (2016) ZnCo2O4 quantum dots anchored on nitrogen-doped carbon nanotubes as reversible oxygen reduction/evolution electrocatalysts. Adv Mater 28:3777–3784. https://doi.org/10.1002/adma.201506197
Prabu M, Ramakrishnan P, Nara H, Momma T, Osaka T, Shanmugam S (2014) Zn-air battery: understanding the structure and morphology changes of graphene-supported CoMn2O4 bifunctional catalysts under practical rechargeable conditions. ACS Appl Mater Interfaces 6:16545–16555. https://doi.org/10.1021/am5047476
Yuvaraj S, Vignesh A, Shanmugam S, Selvan RK (2016) Nitrogen-doped multi-walled carbon nanotubes-MnCo2O4 microsphere as electrocatalyst for efficient oxygen reduction reaction. Int J Hydrog Energy 41:15199–15207. https://doi.org/10.1016/j.ijhydene.2016.06.115
Cheng F, Shen J, Peng B, Pan Y, Tao Z, Chen J (2011) Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat Chem 3:79–84. https://doi.org/10.1038/nchem.931
Zhao T, Gadipelli S, He G, Ward MJ, Do D, Zhang P, Guo Z (2018) Tunable bifunctional activity of MnxCo3−xO4 nanocrystals decorated on carbon nanotubes for oxygen electrocatalysis. ChemSusChem 11:1295–1304. https://doi.org/10.1002/cssc.201800049
Ge X, Liu Y, Thomas Goh FW, Andy Hor TS, Zong Y, Xiao P, Zhang Z, Lim SH, Li B, Wang X, Liu Z (2014) Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS Appl Mater Interfaces 6:12684–12691. https://doi.org/10.1021/am502675c
Li G, Li L, Shi J, Yuan Y, Li Y, Zhao Z, Shi J (2014) One-pot pyrolytic synthesis of mesoporous MnCo2O4 (M = Mn, Ni, Fe, Cu) spinels and its highly efficient high electrocatalytic properties for CO oxidation at low temperature. J Mol Catal Chem 390:97–104. https://doi.org/10.1016/j.molcat.2014.03.012
Yang W, Hao J, Zhang Z, Lu B, Zhang B, Tang J (2014) Synthesis of hierarchical MnCo2O4.5 nanostructure modified MnOOH nanorods for catalytic degradation of methylene blue. Catal Commun 46:174–178. https://doi.org/10.1016/j.catcom.2013.12.018
Gao M, Lu X, Nie G, Chi M, Wang C (2017) Hierarchical CNFs/MnCo2O4.5 nanofibers a highly active oxidation mimetic and its application in biosensing. Nanotechnology 28:485708. https://doi.org/10.1088/1361-6528/aa9135
Hu X, Zhang S, Li X, Sun X, Cai S, Ji H, Hou F, Zheng C, Hu W (2017) Large-scale and template free synthesis of hierarchically porous MnCo2O4.5 as an anode material for lithium-ion batteries with enhanced electrochemical performance. J Mater Sci 52:5268–5282. https://doi.org/10.1007/s10853-017-0767-5
Liao F, Han X, Zhan Y, Xu C, Chen H (2018) Solvothermal synthesis of porous MnCo2O4.5 spindle-like microstructure as high-performance electrode material for supercapacitors. Ceram Int 44:22622–22631. https://doi.org/10.1016/j.ceramint.2018.09.038
Bai Z, Heng J, Zhang Q, Yang L, Chang F (2018) Rational design of dodecahedral MnCo2O4.5 hollowed-out nanocages as efficient bifunctional electrocatalyst for oxygen reduction and evolution. Adv Energy Mater 8:1802390. https://doi.org/10.1002/aenm.201802390
Bazuev GV, Korolyov AV (2008) Magnetic behavior of MnCo2O4.5+δ spinel obtained by thermal decomposition of binary oxalates. J Magn Magn Mater 320:2262–2268. https://doi.org/10.1016/j.jmmm.2008.04.123
Jung KN, Jung JH, Im WB, Yoon S, Shin KH, Lee JW (2013) Doped lanthanum nickelates with a layered perovskite structure as bifunctional cathode catalysts for rechargeable metal-air batteries. ACS Appl Mater Interfaces 5:9902–9907. https://doi.org/10.1021/am403244k
Li J, Zhou N, Wang H, Li H, Xie Z, Chu H, Tang Y, Sun L, Peng Z (2015) Three-dimensional MnCo2O4.5 mesoporous network as an electrocatalyst for oxygen reduction reaction. J Electrochem Soc 162:A2302–A2307. https://doi.org/10.1149/2.0471512jes
Acknowledgements
This research was supported by National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP, Nos. 2015R1C1A1A01051733 and 2018R1C1B6004689).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sasidharachari, K., Yoon, S. & Cho, K.Y. Facile synthesis and evaluation of MnCo2O4.5 nanoparticles as a bifunctional catalyst for zinc-air battery. J Appl Electrochem 50, 907–915 (2020). https://doi.org/10.1007/s10800-020-01432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01432-1